Case study
We fixed a scale-up problem & now use Alphalyse to test all batches
Combining mass spec analyses for mAb characterization and batch-to-batch comparison
After a process scale-up, a mAb product was less stable than in the small-scale production batches. Combining several characterization methods uncovered the cause of the decreased stability and gave our client an overview of their antibody’s structural features.
One of our clients, a European pharmaceutical company, is developing immunotherapeutic antibodies for cancer treatment. The company has several drug candidates in pre-clinical and phase 1/2 trials.
Characterization design
The client has used Alphalyse for individual analyses of early-stage products, including protein identification by nanoLC-MS/MS.
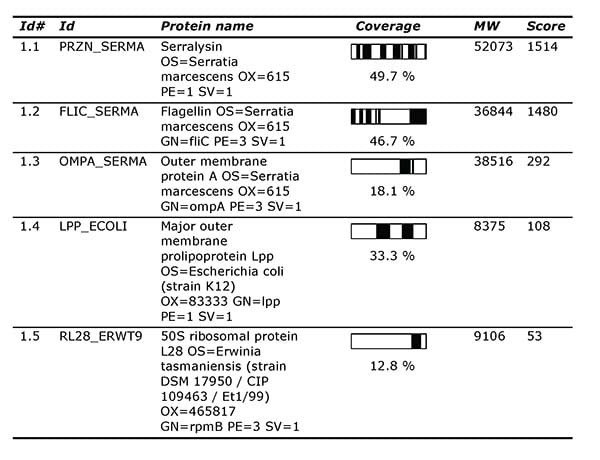
List of protein hits from Protein ID report.
However, after a process scale-up, our client observed that one of their antibody products showed decreased stability. The client therefore asked us to perform a combination of mass spectrometry analyses for product characterization, including intact mass analysis, peptide mapping, N- and C-terminal sequencing, and antibody disulfide bonds analysis, to identify the structural features causing issues with the mAb’s stability.
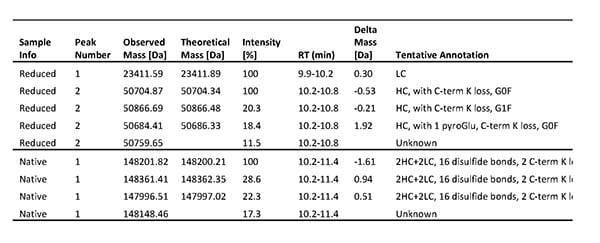
Results from Intact mass analysis of one DS batch. Both the reduced and native antibody was analyzed.
Based on our findings, the client could intelligently optimize their process to overcome the stability issue. The client now uses our characterization program as a quality check for all future product batches.
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.