Case study
Troubleshooting HCP ELISA results using LC-MS and ELISA-MS™
Characterization of HCP ELISA kit standard
When our client suddenly experienced significant changes in the Host Cell Protein (HCP) levels of identical drug samples, ELISA-MS™ coverage analysis and quantitative LC-MS revealed that the changes were due to the recent implementation of a new HCP ELISA kit standard. The client is now developing a custom ELISA standard that better fits the HCPs in their drug substance.
Following the sudden introduction of a new HCP ELISA kit version by the vendor, our client, an EU-based biotechnology company manufacturing vaccines and cancer immunotherapies, observed an abrupt 20-fold decrease in the HCP levels measured on an identical drug substance sample.
The process
The client contracted Alphalyse to investigate the reason for this sudden change. As part of the analysis, we performed an ELISA-MS™-based coverage analysis on the kit itself, as well as an LC-MS evaluation of the suitability of the HCP ELISA standard, since we had a hunch this could be the problem.
Results of our analysis
A combination of the ELISA-MS™ coverage analysis and quantitative SWATH-based HCP-MS found that the new kit standard contained disproportionately high amounts of 5 HCPs found in the drug sample as can be seen in this graph:
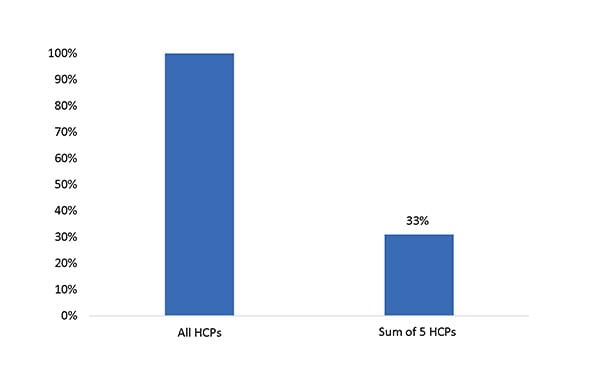
HCP content of harvest sample measured by LC-MS
In addition, the ELISA kit antibodies were overly sensitive to these exact same 5 HCPs.
Unfortunately, this made the ELISA calibration slope shift (see the calibration curve example below), resulting in a misinterpretation by the ELISA of the total amount of HCPs in the drug sample.
The client has subsequently contracted Alphalyse to characterize a new custom-developed ELISA standard to evaluate if it is a better fit for the HCPs in their drug substance.
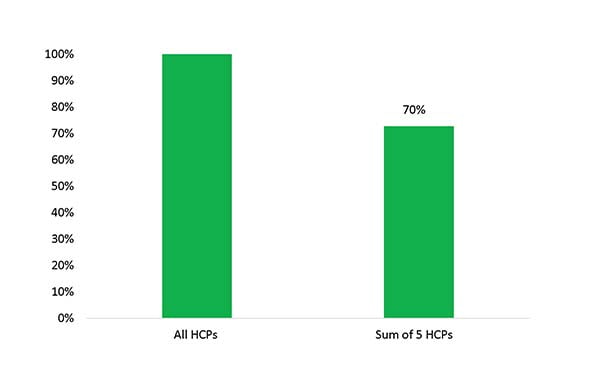
Sensitivity of immunocapture to specific HCPs in harvest sample
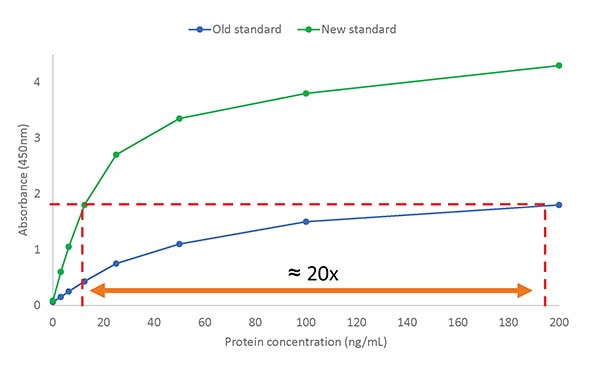
ELISA calibration curves
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.