Case study
Revealing HCPs not detected by ELISA in antibody-drug product
Supplement ELISA with mass spectrometry data - and check for problematic HCPs
HCP ELISA and an initial mass spectrometry (LC-MS) analysis of our client’s monoclonal antibody (mAb) process samples indicated it was very pure. None of the methods identified Host Cell Proteins (HCPs) in 2 of 4 purification steps. However, by lowering the LLOD, it was possible to detect 4 HCPs of potential concern for product stability and patient safety.
One of our clients is a drug discovery company that develops monoclonal and bispecific antibodies to treat cancer and autoimmune conditions.
Usually, this client uses commercial ELISA kits for Host Cell Protein (HCP) analysis, which provide a summed value of the sample’s total HCP content. However, the client was unsure if their ELISA reported all HCPs present in their drug substance. They also wished to use an orthogonal HCP analysis to look for specific problematic impurities.
Unlike ELISA, a liquid chromatography-mass spectrometry (LC-MS)-based orthogonal assay can identify and quantify individual HCPs. Absolute quantification of individual HCPs can be achieved by multiple reaction monitoring (MRM) with a suitable internal standard.
Step 1:
LC-MS assay using a quantitative digest method showed no HCPs
The client sent us four samples from different purification steps to produce their monoclonal antibody (mAb) product expressed in E. coli. The primary aim was to get an overview of HCP clearance throughout the purification process.
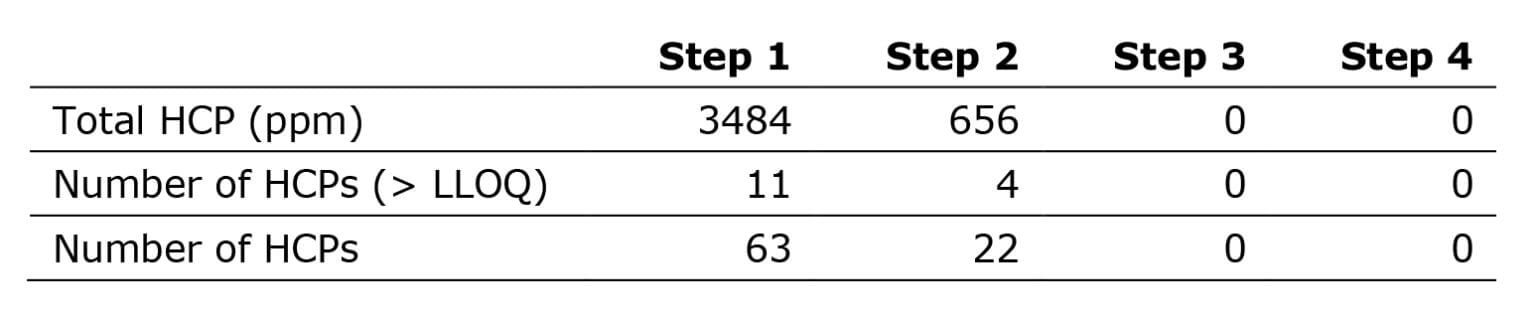
Initially, we applied our quantification digestion protocol (the quantitative assay), a well-established method that works well for all sample types and expression systems. The analysis detected more than 20 HCPs in process steps 1-2, whereas analysis of steps 3 and 4 resulted in no HCPs above limit-of-detection (LOD).
Most MS-based approaches have detection limits at the sub-ppm level. However, HCPs with e.g., enzymatic activity can significantly degrade a drug substance or excipient while being present below sub-ppm concentration levels and going undetected by standard LC-MS. For this reason, the client requested additional testing of their product samples.
Step 2:
Native digest substantially lowered the limit of detection (LOD)
To check for HCPs below 1-5 ppm, we applied a semi-quantitative, native digest method to lower the limit of detection (LOD) substantially to around 0.1 ppm. This method digests HCPs for LC-MS detection while leaving the mAbs largely intact.
Following the digest procedure, a precipitation step removed most undigested mAbs. This step thus increased the amounts of digested HCPs loaded onto the LC-MS instrument, substantially expanding the assay’s sensitivity. As a result, we could now detect over 200 HCPs in step 2, and more than 20 HCPs in steps 3 and 4, which had previously seemed pure.
Using this data, we evaluated the observed HCPs for problematic properties and found 4 HCPs of potential concern for product stability and patient safety – even below one ppm.

Top 10 HCPs identified in the process steps 2-4 with the semi-quantitative, native digest method. Instead of ppm, the table shows the number of detected peptides originating from the proteins. HCPs of potential concern are marked in bold.
Following changes to the client’s purification process, a new DS sample showed a reduction of both the number of HCPs and ppm. Of the four HCPs of potential concern quantified above LLOQ in the first batch, none were found in the new sample.
This case demonstrates the necessity of using a highly sensitive LC-MS assay to document clearance of impurities in mAbs, since HCPs present below sub-ppm concentration levels may go undetected by both ELISA and standard LC-MS.
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.