Literature
How to measure the consistency of viral proteins in therapy products
- and why it’s important
By Sai Sindhu Thangaraj, Protein Analysis Scientist, and Ejvind Mørtz, COO, Alphalyse
Cell and Gene Therapy (C>) products are evolving rapidly, and hundreds of projects have now entered clinical trials. There are more than two hundred clinical trials for recombinant adeno-associated (rAAV) vector-based gene therapies alone, and the FDA has already approved two products. Both the FDA and EMA are asking for greater quality control and process consistency [1-4]. In this regard, monitoring viral protein consistency between batches is essential.
In 2020, the FDA issued specific guidelines on Chemistry, Manufacturing, and Control (CMC) – Information for Human Gene Therapy Investigational New Drug Applications (INDs). The guidelines recommend:
“that you have tests in place to measure levels of residual contaminants,
including “helper” virus contaminants (i.e., infectious virus, viral DNA, viral proteins),
host cell proteins, and exogenous nucleic acid sequences.”
In addition, the FDA’s Peter Marks stated last year that developers need to “follow the product over time so that one understands what one is dosing, and how much one is dosing in terms of potency or of the product.”
An example of a critical potency factor is the amount of active viral capsids present. Because of its importance to potency, it has become an essential attribute to monitor.
Why is viral protein consistency between drug batches important?
For monitoring C> consistency, it is becoming essential to analyze the correct expression of the capsid proteins. Monitoring is important to avoid the production of capsids not containing the vector genome (an empty vector), truncated versions, or those with contaminant genetic material. Factors to investigate include size, peptide sequence, and post-translational modifications since they affect viral assembly and capsid potency.
Likewise, the proper function of the drug requires correct genome load and viral protein ratio. An accurate viral protein load determines the appropriate folding of the virus needed to encapsulate the DNA correctly. In contrast, partially filled or truncated vectors have no activity.
The amount of partially filled or truncated viral vectors can significantly impact the drug dose and potentially contribute to inducing inflammation if delivered to patients in high doses. An additional complicating factor is the tendency of different cell lines to produce varying viral protein ratios.
The factors mentioned above suggest that it is critical to analyze the integrity of the viral capsids and genome because of their critical role in transgene integrity and product safety and efficacy. Critical quality attributes (CQAs) should include capsid purity and impurities, genome integrity, and process-related host cell protein impurities.
Manufacturing consistency is vital to achieving uniform C> product safety and potency with precise dose determination.

The complex nature of cell and gene therapy products makes them challenging to analyze and characterize.
Contemporary viral vector-based C>s utilize vectors like retroviruses, adenoviruses, or adeno-associated viruses. These viral vectors consist of a protein shell or capsid enclosing the gene therapy product (DNA) and other core proteins. The vectors are packaged by transformation into cell lines and produced by cell culture. The viral vectors are then purified from the cell suspension by different processes and delivered to patients.
The production process results in C> drugs that may consist of a complex mixture of the gene therapy drug, residual host cell proteins, viral vector proteins, and possibly other components added during cell culturing and affinity purification, including bovine serum, human albumin, antibodies, etc. The complex composition of the drug substance challenges how the process residuals and viral proteins are analyzed.
C> products are complex drug types challenged by the regulatory guidelines to verify viral capsid purity, residual protein clearance, and product consistency.
Why available analytical methods are not ideal for C>s
The FDA guidelines clearly state what needs to be measured, but they do not indicate which methods should be used. While the establishment of CQAs is key, many potential CQAs are not identified yet or not measured adequately due to a lack of proper analytical tools. Rapidly evolving manufacturing methods further compound the challenge and require next-generation analytical methods.
Some of the methods most often used to characterize the viral proteins in C> products include [5]:
- HPLC
- qPCR
- Optical Density
- Analytical Ultracentrifugation
- Size Exclusion Chromatography – Multi-angle light scattering (SEC-MALS)
- Mass Spectrometry (LC-MS)
- Capillary Isoelectric Focusing (cIEF)
The table below summarizes the main pros and cons of each method and their suitability for C> analysis:
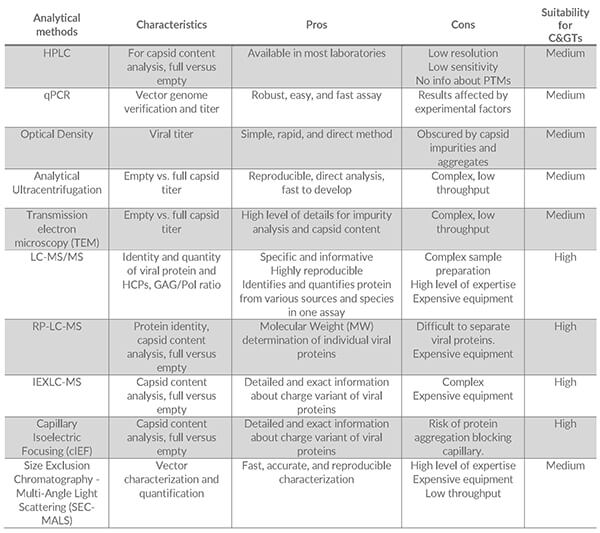
Some of the most commonly used techniques are discussed in more detail below:
qPCR can determine DNA levels and measure the amount of viral protein. However, DNA levels do not provide information about full vs. empty capsid ratios, which are essential for determining the correct drug dose and viral content dosing.
Optical density, ultracentrifugation, and transmission electron microscopy can determine viral titers and empty vs. full capsid titers, although using only one of these methods is not advisable. Accuracy and precision may be low and may lack information on the specific amount of the components involved in the C> products, e.g., viral protein.
Mass spectrometry allows specific characterization of the drug substance for C> products. LC-MS is an all-round method that can identify and characterize variants of the viral proteins. Furthermore, novel approaches can quantify viral and host cell proteins simultaneously, such as determining the Gag to Gag-Pol ratio in lentiviral vectors.
Which method do the regulatory authorities recommend?
C> producers have freedom of choice for the viral protein analysis as long as they can demonstrate that the method is fit-for-purpose. Due to the complex composition of C> drugs, which contain proteins from host cells, viral proteins, and other cell culture-derived sources, a multiplex analysis method that simultaneously identifies and quantifies multiple proteins and their variants would be optimal.
For this purpose, methods based on chromatography combined with mass spectrometry (LC-MS) are ideal. They can be used to study viral proteins, residual host cell proteins, and proteins from other sources.
Alphalyse has used mass spectrometry to measure viral protein and host cell proteins in several C> customer projects based on lentivirus, adenovirus, and adeno-associated viruses. One such project used Spodoptera frugiperda (SF9) cells infected with a baculovirus (AcMNPV) to manufacture AAV5-based drugs. The LC-MS analysis discovered a considerable variation in the viral protein quantity between batches.
The advantages of LC-MS for analyzing process-related residuals in C> development and manufacture have thus already been clearly demonstrated.

References
[1] World Medical Innovation Forum: “FIRESIDE Interview with Peter Marks, FDA,” 2021
[2] Ned Pagliarulo: “FDA seeking more consistency from cell, gene therapy developers, top official says,” Biopharma Dive 2021
[3] European Medicines Agency: “Guideline on Immunogenicity assessment of therapeutic proteins,” 2017
[4] U.S. Food & Drug Administration: “Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs),” 2020
[5] Alphalyse: “Infographic: Tomorrow’s Analytical Techniques – for ensuring consistent C> products,” 2022
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.