Literature
Ensuring process consistency and product quality in C>s
By Ejvind Mørtz, COO, and Jette Friis Thirup, Head of Business Development, Alphalyse
The cell and gene therapy (C>) industry is developing rapidly, with an increasing number of products entering clinical trials and receiving marketing approval from the FDA each year. However, the processes for manufacturing complex C>s are still under development and demand improved analytical tools to ensure process consistency and product quality.
Like more classic biologics, C> companies use host cells to produce new therapeutics and viral vectors. The manufacturing process generates process-related impurities that may reduce the drug's efficacy. And some may even cause toxicity and long-term immunogenicity in the patients.
Therefore, legal authorities like the FDA and EMA require investigation, monitoring, and documentation of residual protein levels for all biologics, including C>s.
More traditional biologics, such as mAbs, have manufacturing processes that are well-defined and supported by a panel of established analytical test methods. However, the manufacturing processes for complex C>s are still in development. It thus demands improved analytical tools to ensure process consistency and product quality.
FDA tightens policies ahead of an expected boom in cell and gene therapies
In 2019, Bravery et al. [2] reviewed 22 C>s approved in the EU and US and concluded that comparability, consistency, and impurities issues were the major regulatory objections raised. By 2025, the FDA foresees they will be reviewing and approving between 10 and 20 C>s each year [3, 4]. Consequently, the FDA increasingly requires C> developers to manufacture these complex products more consistently. To this end, the agency has delayed several product approvals, asking developers to improve the documentation to show product consistency [5].
Since C>s are relatively new in biopharma, no specific guidelines existed for this class of biologics until recently. In 2018, the EMA published “Guidelines on the quality, non-clinical and clinical aspects of gene therapy medicinal products” [6]. Likewise, the FDA issued its guidelines on “Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs)” in 2020 [7].
The FDA guidelines include a specific chapter on process-related impurities, which states: “We recommend that your manufacturing process is designed to remove process- and product-related impurities and that you have tests in place to measure levels of residual contaminants.”
It goes on to say: “These include cell-substrate proteins, extraneous nucleic acid sequences, helper virus contaminants (i.e., infectious virus, viral DNA, viral proteins) and reagents used during manufactures, such as cytokines, growth factors, antibodies, selection beads, serum, and solvents.”
Though the guidelines clearly state what to measure, it does not say which measurement methods to use.
Why the complexity of C>s poses analytical challenges
Traditional biologics are typically manufactured by expressing one protein in a single cell line and purifying it, using steps to detect and monitor impurities until their concentrations are low enough in the final drug product to meet regulatory requirements [1]. However, C>s are more complex and can consist of a mixture of proteins from different sources, e.g., the viral vector such as Adeno Associated Virus (AAV), Adenovirus, or Lentivirus, the production and packaging cell line, e.g., insect Sf9 cells, human HEK293cells, or more rare cell lines like Murine PG13.
Furthermore, manufacturing may employ various proteins from multiple sources and organisms during cell growth and harvest. While the cell lines used to produce the therapeutics are generally human [1] or from other mammals, the proteins added to the cell culture usually come from other species, such as cows – e.g., bovine serum – or bacteria – e.g., benzonase.
The mixture of many different proteins, species, and components makes it difficult to use classical impurity assays. One example is the ELISAs that only measure proteins from one species.
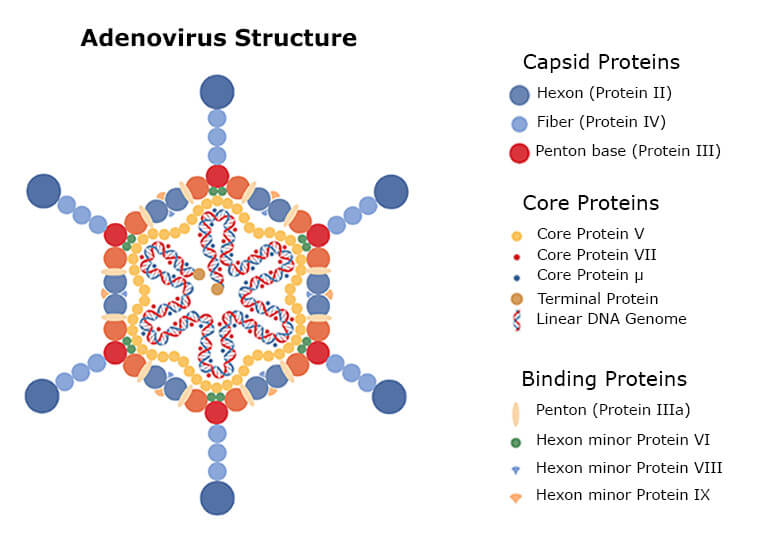
Figure 1: The structure of adenovirus
Traditional analytics fall short when used for C>s
Immuno enzyme-linked immunosorbent assays (ELISAs) have been the conventional approach in biopharma for analyzing residual proteins. Mainly due to their speed, sensitivity, and ease of use. Other advantages of ELISA include the high sample capacity, making it possible to analyze many samples simultaneously.
However, off-the-shelf ELISAs only measure proteins from one species, not across species. There is no single commercial ELISA capable of detecting the heterogeneous mix of proteins from different species used in the production of C>s.
The host cell proteins that arise from using a human cell line are not very immunogenic in the rabbits and goats typically used for raising ELISA antibodies. Since ELISA only measures proteins that generate an antibody response, ELISAs tend to have low coverage of human proteins in C>s. They may therefore fail to detect a substantial proportion of the process-related residuals.
To address the problem of low coverage, manufacturers often develop a process-specific ELISA. However, antibody development and assay validation typically take one-and-a-half to two years. Such a timeframe can be too long for novel gene therapies and vaccines that are often fast-tracked by regulatory authorities to treat severe, life-threatening conditions. As gene therapies can be one-shot cures, being first to market could effectively mean owning the whole market [8, 9], so speed is of the essence.
The emergence of orthogonal analysis methods
Lately, there has been a steady increase in industry regulators requesting that submissions include data detailing host cell protein and viral protein quantification obtained with methods orthogonal to ELISA [1].
The result is that the industry has come to see mass spectrometry analysis (LC-MS) as an orthogonal tool for documenting residual proteins in C>s. The method identifies and quantifies protein impurities from various sources and species in a single, highly reproducible assay. Therefore, LC-MS is well-suited for documenting process-related impurities in complex biopharmaceuticals like C>s. It will likely become the gold standard for monitoring and reporting residual proteins in C>s.
References
[1] Biopharm International Editors: “A Novel Method for Host Cell Protein Analysis.” Biopharm International 2020
[2] Bravery CA, Ball O, and Robinson S. EU market authorization strategy: “Lessons from the first 22 ATMP submitted to the EMA.” Cell & Gene Therapy Insights 2019
[3] Lukashev et al.: ”Viral Vectors for Gene Therapy: Current State and Clinical Perspectives.” Biochemistry (Moscow), 2016
[4] Hernandez Bort et al.: ”Challenges in the Downstream Process of Gene Therapy Products.” American Pharmaceutical Review, 2019
[5] Johnathan Gardner: “FDA gene therapy holdups suggest closer scrutiny by agency.” Biopharma Dive 2020
[6] “Guideline on quality, non-clinical and clinical requirements for investigational advanced therapy medicinal products in clinical trials.” European Medicines Agency 2019
[7] “Chemistry, Manufacturing, and Control (CMC) information for human gene therapy investigational new drug applications (INDs). Guidance for industry.” US Food and Drug Administration 2020
[8] Ejvind Mørtz and Todd Stawicki: “A Smarter Way To Remove Host Cell Protein Contamination From Gene Therapies.” Technology Networks, Biopharma 2020
[9] Niamh Kinsella & Clare Blue: “Demonstrating Comparability of AAV Gene Therapy Products During Clinical Development: Managing the Link Between the Product and the Process.” Cell & Gene Therapy Insights 2021
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.