Literature
How to select an HCP ELISA with sufficient coverage
- for regulatory documentation
August 25 2021, by Ejvind Mortz, COO, Alphalyse
Question:
How do I choose the best ELISA kit for my biologic? In other words, I would like to select the impurity assay with the highest coverage of the process HCPs, not only for harvest samples but also for the HCP impurities in the purified drug substance.
Answer:
Host Cell Protein (HCP) analysis is essential in development and regulatory documentation of biologics.
Since specific HCPs are problematic, either as a potential risk factor for immunogenicity in patients or by affecting drug stability, biologics should be as free of residual HCPs as possible [1, 2].
Historically, most companies performed HCP analysis using anti-HCP ELISAs raised by immunizing animals with a protein lysate from the host cell line [1, 2].
Today, most companies also use orthogonal methods for HCP characterization, including liquid chromatography mass spectrometry (LC-MS), to quantify individual and potential problematic HCPs. Orthogonal methods are especially beneficial when there is no commercial kit on the market, or it is impossible to develop a good HCP ELISA.
Still, HCP ELISAs are valuable during process development, characterization, and validation [2].
Selecting the best ELISA kit: HCP antibody coverage analysis
Before a commercial HCP ELISA kit or a custom-developed HCP ELISA is considered sufficient and fit-for-purpose, you must perform an HCP antibody coverage analysis. This analysis determines how completely a population of polyclonal antibodies recognizes the population of HCPs, as stated in USP 39 [2].
Thus, comparing the coverage of various ELISAs – kits or custom – allows you to pick the best and most fitting ELISA for your drug.
Traditionally, you had to choose between two methods for HCP coverage evaluation. These were 2D gels followed by Western Blotting or immunoaffinity purification and 2D gel analysis.
However, both methods have widely known limitations, including manual and variable spot counting in gels [2, 3]. This led to improved coverage evaluation methods, such as ELISA-MS, which provides the name and identity of each HCP covered by the ELISA kit [4].
Coverage analysis with only 0.5 mg antibody – without a mock cell line
ELISA-MS combines ELISA-based immunocapture in the 96-well plate with LC-MS. The result is a list of individual HCPs in a drug sample recognized by the specific ELISA antibodies [4].
Where 2D gel-based and immunoaffinity chromatography-based coverage methods evaluate the antibodies outside of the ELISA plate, ELISA-MS mimics the conditions of the HCP-ELISA. Thus ELISA-MS captures the HCPs by the anti-HCP antibodies under the same conditions as the ELISA in the 96-well plate.
2D gel-based methods rely on spots in a gel to provide a total number of covered HCPs. If you only utilize this method, you cannot know which HCP the spot represents. And perhaps worse, you do not see the coverage of each HCP.
Moreover, column-based methods often need 10-15 mg ELISA antibodies. Whereas ELISA-MS only requires 0.5 mg antibody for running HCP coverage analysis and thus saves your valuable ELISA antibodies.
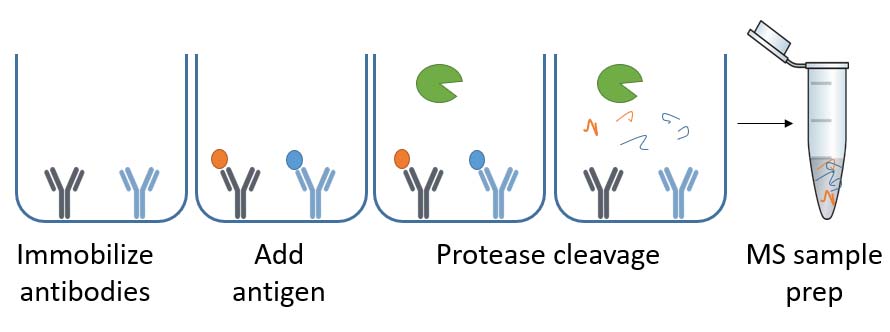
Illustration of the ELISA-MS™ method
With ELISA-MS, you can perform coverage analysis on an early process sample containing the drug-protein. Thus, you do not have to generate a mock cell line with the risk of having another HCP profile than your drug samples. This way, you can perform coverage analysis without investing time and money in developing the mock cell line.
Finally, when you get the lists of the individual HCPs covered in your process and the HCPs in the purified product, you will know if the HCP-ELISA detects the individual impurities in the product. Therefore, many consider ELISA-MS the best method for determining the coverage of ELISA kits.
You are free to use the best method – both for coverage and HCP analysis
We often get the question: Which coverage method do the regulatory authorities require?
The answer is that you have freedom of choice for the HCP analysis methods as long as you can demonstrate that the method is scientifically sound.
Are you interested in learning more about different HCP coverage methods to select the best ELISA kit? Then check out this webinar with HCP expert Rikke Raaen Lund:
References
[1] Bracewell et al.: “The Future of Host Cell Protein (HCP) Identification During Process Development and Manufacturing Linked to a Risk-Based Management for Their Control.” Biotechnology and Bioengineering. 2015
[2] U. S. Pharmacopeia: “USP 39 Published General Chapter <1132> Residual Host Cell Protein Measurement in Biopharmaceuticals”. 2016
[3] Zhu-Shimoni et al.: “Host Cell Protein Testing by ELISAs and the Use of Orthogonal Methods. “Biotechnology and Bioengineering. 2014
[4] Pilley et al.: “A novel approach to evaluate ELISA antibody coverage of host cell proteins-combining ELISA-based immunocapture and mass spectrometry.” Biotechnology Progress. 2020
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.