Advanced therapies and vaccines client stories
Our aim is to provide you with the best protein analysis service by providing access to our service-minded team of highly experienced and skilled scientists, hand-picked from leading universities and companies. Together, they cover most fields of protein chemistry, mass spectrometry and bioinformatics. They also apply top-of-the-line equipment and are experts in interpretation of its data for biopharmaceutical protein characterization.
Client stories
Webinars & videos

Exclusive Q&A with Bryant McLaughlin, CMC Executive, USA
33:03

Analyzing vaccine purity – without an HCP ELISA
46:45

ELISA reagent characterization using advanced LC-MS methods
44:09

LC-MS HCP assay validation and GMP release testing for complex samples
50:34

#Rethink your Host Cell Protein strategy
33:30

The principles of mass spectrometry-based HCP Analysis
22:50

How to monitor impurities and ensure consistent gene therapies
34:45

Moving LC-MS analysis into a GMP environment
39:46

Are you ready for FDA questions about impurities?
35:39

Video: What is the root-cause of polysorbate degradation?
1:45

Video: ICH Q8B characterization of complex products
1:50
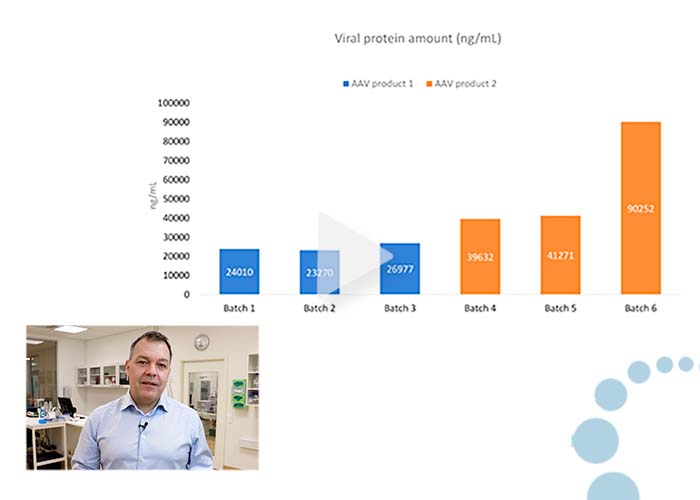
Video: Analysis of residual protein in viral products
1:58

Video: Expression systems without a commercial ELISA kit
2:04

Video: Monitoring process-added enzymes using an MRM assay
1:45
Video: Analysis of bacteriophage product
1:52

Video: Inactivated virus vaccine, Vero cells, and quantification of protease
2:08

Video: Analysis of advanced
therapies
2:34
Video: GMP-validated HCP analysis based on LC-MS
1:35
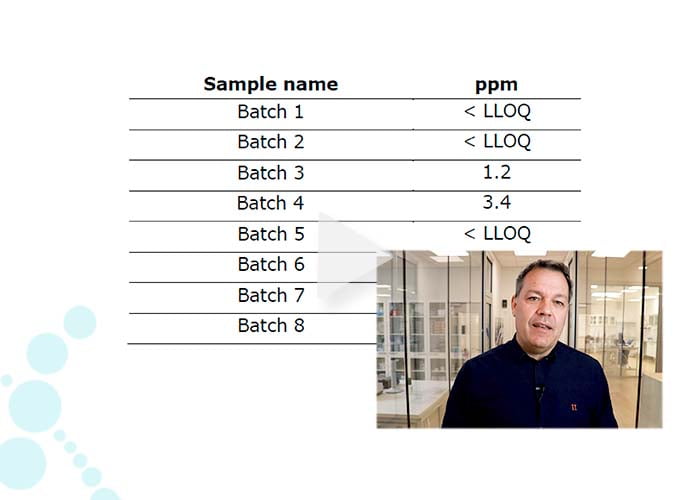
Video: Residual biocatalysis in small molecule API
1:56

Video: Qualification report used for IND application
2:07
Literature
Whatever ELISA-related challenge or question you may have, we are here to help you solve it. One of our protein analysis experts will discuss the best analysis approach or method for your project by email or online meeting – without obligation.

Infographic: Towards consistent C>s

Poster: HCP Coverage by Immunocapture and LC-MS/MS

Brochure: Coverage analysis using ELISA-MS™
Article: A smarter way to remove host cell protein contamination from gene therapies

Article: Monitoring process-related impurities in biologics - host cell protein analysis

Poster: Sensitive MS-assay for detection of polysorbate-degrading HCPs in mAb products

Article: A novel method for host cell protein analysis

Poster: ID & quantify individual HCPs using SWATH® LC-MS

Brochure: Mass spectrometry-based HCP analysis

PDF: Standard HCP assay qualification protocol

White paper: HCP analysis of downstream process samples by mass spectrometry
Poster: Sensitive MS-assay for detection of polysorbate-degrading HCPs in mAbs
Brochure: Coverage analysis using ELISA-MS™
Poster: ID & quantify individual HCPs using SWATH® LC-MS
Article: A novel method for host cell protein analysis
Article: A smarter way to remove Host Cell Protein contamination from gene therapies
PDF: Standard HCP assay qualification protocol
White paper: HCP analysis of downstream process samples by mass spectrometry
Brochure: Mass spectrometry-based HCP analysis
Poster: HCP Coverage by Immunocapture and LC-MS/MS
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.