Case study
Quantifying lentivirus and residual proteins in one assay
"We use the Gag/Gag-Pol ratio to monitor viral capsid formation"
The cell & gene therapy division of a UK-based pharma company was initially looking for a host cell protein (HCP) assay to document the individual residual proteins in their lentivirus vector-based gene therapy. While examining the results, the client discovered that they could use the MS assay to monitor individual viral proteins. It was also possible to detect essential proteins for the viral capsid structure and assembly.
The client had given up on finding an ELISA assay and instead came to us to develop a mass-spectrometry-based assay. The assay produced a list of proteins, separated into HCPs from HEK293T cells and those from the growth medium. Table 1 shows some of the detected viral proteins in the five analyzed batches.
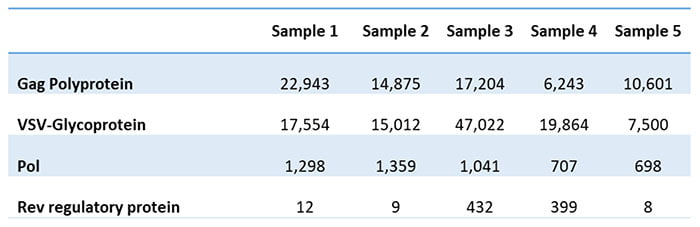
Table 1: Viral proteins detected in five batches
The client could also determine the Gag to Gag-Pol ratio based on the results. This ratio is essential for the successful assembly and maturation of viral particles. Typically, the critical ratio for producing infectious viral particles is 20:1. Attempts to vary the 20:1 polyprotein ratio may decrease virus infectivity and stability. The client had unsuccessfully tried to estimate the ratio using other methods but now gets it from the residual protein analysis.
In addition, the analysis was able to pool the individual viral quantities to give the total viral protein in ng/mL in each sample (Figure 1). The study showed that sample 3 varied significantly from the others. As a result, the client began looking into possible causes of these batch variations.
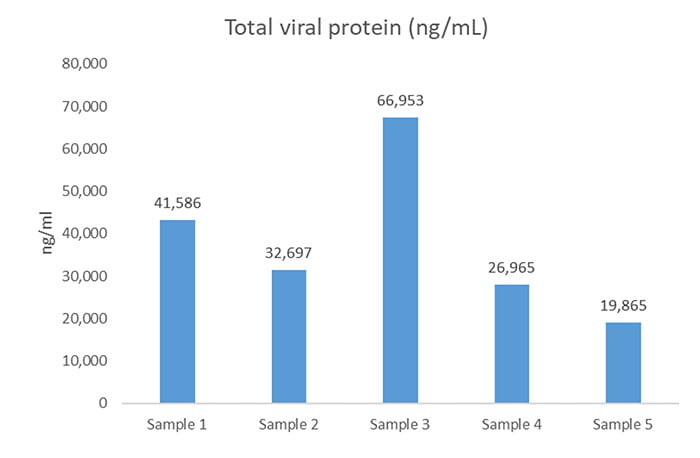
Figure 1
Bonus: Easy measurement of the growth media
In developing their gene therapy, the client utilized lentiviral particles packaged in a HEK293T producer cell line. These two different protein sources create complexity which is compounded by growing the HEK293T cells in complex cell media with human serum albumin (HSA). The complex protein mixture further complicated the analysis of process-related impurities in the lentivirus-based gene therapy.
In addition to analyzing the batches of lentivirus gene therapy, the assay also analyzed the growth medium on its own. The analysis revealed the following HCPs in the samples (Table 2), which trace back to the growth medium.
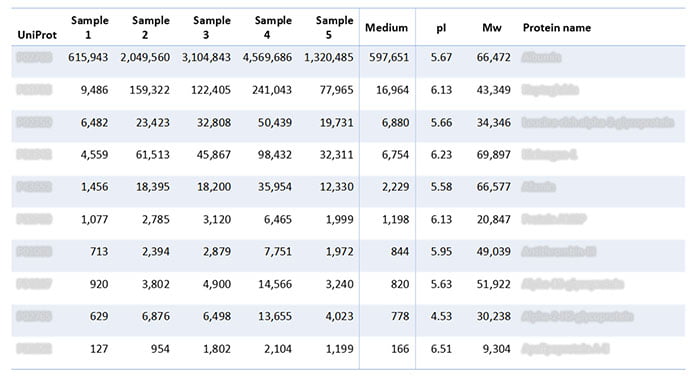
Table 2: HCPs identified in batches of lentivirus gene therapy
The results enabled the client to thoroughly document their manufacturing process for internal optimizations and regulatory documentation.
Find out more about the gene therapy product analysis here
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.