Literature
Why worry about process-related impurities in C> development?
by Ejvind Mortz, COO, and Martin Rask Johansen, Client Relations Manager, Alphalyse
In response to the many cell and gene therapy (C>s) products in development [1], the FDA and EMA recently published new guidelines for developers advising greater monitoring of process-related impurities. However, many C> developers experience problems removing contaminants and properly characterizing the product.
What are impurities in complex biologics?
During the development and production of complex biologics such as cell & gene therapy products (C>s), process-related impurities co-purify along with the product. The EMA Q6B guidelines [2] divide these impurities into three subtypes depending on their origin:
- Cell substrate-derived (host cell proteins, host cell DNA, etc.)
Cell culture-derived (antibiotics, IPTG, DTT, growth factors, etc.)
Downstream-derived (enzymes, buffer components, etc.) - The cell substrate-derived impurities include host cell proteins (HCPs), of which some are of concern if they are present in the final product. They include lipases, ubiquitin, peptidylprolyl isomerase, heat shock protein, cathepsin, and serine protease [3].
The cell culture-derived impurities include proteins added to the growth medium, e.g., fetal calf serum, human albumin, cytokines, and enzymes used for harvesting and lysing cells such as TrypLE.
Downstream-derived impurities consist of agents used to express and purify biological protein products. These include the Benzonase nuclease, affinity column antibodies for product capture, enzymes for site-specific PEGylation, aminopeptidase, and Protein A. Typical residuals also include Tris, carriers, ligands, Tween/Polysorbate, DCA, TCEP, heavy metals, solvents, Triton-X, antifoaming agents, PEI, TFA/Acetate, Imidazole, etc.
Why process-related impurities are a concern
Investigating residual protein levels is crucial, especially in the final product, because the residuals can influence the stability and efficacy of the active ingredient and may also pose a risk to the patient’s health:
Such ‘problematic’ proteins may…
- Affect drug efficacy by reducing protein stability and potency,
and/or - Induce immunotoxic effects and immunogenic reactions in patients
-even in sub-ppm amounts.
The foreign, residual protein increases the risk of triggering an immune response in the patient.
Most host cell proteins (HCPs) have the potential to generate an immune response in humans due to the genomic differences between the human patient and commonly applied protein production hosts, such as E. coli, yeast, mouse myeloma cells (NS0), and Chinese Hamster Ovary cells (CHO). For example, Wang et al. showed that a reduction in HCPs correlated with a decline in the release of specific inflammatory cytokines [4].
There is also a risk that some unwanted HCPs, similar to human homologs, may have a biological function in humans resulting in potential side effects [4].
To summarize, it is essential to monitor residual proteins since they influence:
- The product quality by proteolysis, particle formation, or enzymatic modification;
- The process consistency and, therefore, the ability to produce a pure product;
- The patient safety by inducing immunogenicity or decreasing the activity of the biologic’s active ingredient.
With the development of sensitive analytical methods for residuals, the FDA and EMA are increasingly interested in data that describes the efficiency of the purification process for C>s.
Hence, an analytical method is needed to detect the exact process-related impurities in your product – throughout process development and the final drug substance.
It is challenging to monitor problematic residual proteins using ELISA
The FDA increasingly asks C> developers to document residual protein clearance before their biologic goes into late-stage clinical trials [5].
The problem is that traditional methods like ELISA and HPLC are limited by being unable to distinguish between impurities (low resolution) and insufficient sensitivity (limit of detection), respectively. Since the residuals are present at low-ppm levels, you need a reproducible approach with a high sensitivity to get a detailed overview of the residual protein clearance [6-7].
Furthermore, since the products are complex and contain proteins from multiple sources and species, a standard commercial ELISA cannot measure all these process-related impurities. An alternative is to develop a process-specific ELISA to cover the residual protein in these products, but this is costly, time-consuming, and challenging.
Orthogonal method for analysis of residual protein impurities
A typical challenge is the detection of protein residuals in products based on adenovirus expressed in a human cell line, e.g., HEK293 or A549 cells, grown on a cell substrate containing bovine serum albumin.
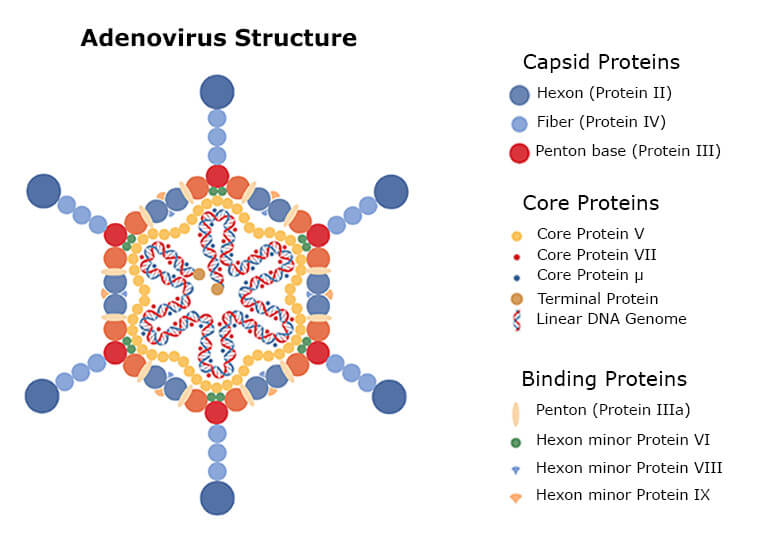
Adenoviruses comprise a protein capsid with different proteins enclosing the DNA and core proteins (see the figure above). When the virus is reproduced and purified from the human host cells, the virus drug substance contains small amounts of residual human proteins. Therefore, the high number of different proteins in the drug requires characterization by a highly sensitive method before clinical administration [8-10].
The best analysis method for this mixture is SWATH LC-MS (liquid-chromatography mass spectrometry), based on data-independent acquisition (DIA), as it can both quantify and identify the different protein impurities. The LC-MS analysis is highly reproducible, robust, and thus suitable for residual protein and HCP analysis.
The analysis provides A) the total amount of residual proteins in ng/ml, B) a list of identified residual proteins and their amounts, and C) a list of identified viral proteins and their amounts.
Mass spectrometry does not rely on animal immune responses; it can identify and quantify individual proteins. The method is thus ideal for documenting residual proteins from different expression organisms that give rise to process-related impurities in complex C> biopharmaceuticals [11-12].
Benefits of using SWATH LC-MS for analyzing process-related impurities in C>s
- Highly reproducible identification and quantification;
- Low interference from the drug substance (which is present in large amounts) on the signal of low abundant residuals;
- The ability to directly compare batches as a tool for quality control after process scale-up;
- High throughput sample handling makes it possible to assess residuals from multiple manufacturing steps quickly;
- Increased sensitivity makes it possible to quantify low ppm levels.
With these advantages, SWATH LC-MS is therefore ideal for analyzing process-related residuals used in biologics development.
You can find more literature on how process developers benefit from using SWATH LC-MS here >>

References
[1] Grand View Research: “Cell And Gene Therapy Clinical Trials Market Size, Share & Trends Report,” 2021
[2] European Medicines Agency: “Guideline on Immunogenicity assessment of therapeutic proteins,” 2017
[3] Marissa Jones, Nisha Palackal, et al.: “High-risk host cell proteins (HCPs): A multi-company collaborative view,” Biotechnology and Bioengineering 2021
[4] Wang et al.: “Host Cell Proteins in Biologics Development: Identification, Quantitation and Risk Assessment,” Biotechnology and Bioengineering, 2009
[5] Johnathan Gardner: “FDA gene therapy holdups suggest closer scrutiny by agency,” Biopharma Dive, 2020
[6] Zhu-Shimoni et al.: “Host Cell Protein Testing by ELISAs and the Use of Orthogonal Methods,” Biotechnology and Bioengineering, 2014
[7] Bracewell et al.: “The Future of Host Cell Protein (HCP) Identification During Process Development and Manufacturing Linked to a Risk-Based Management for Their Control,” Biotechnology and Bioengineering, 2015
[8] Krawitz et al.: “Characterization of Residual Host Cell Protein Impurities in Biotherapeutics, “ Analytical Characterization of Biotherapeutics, 2017
[9] Rux et al.: “Adenovirus structure,” Human Gene Therapy, 2005
[10] Jin et al.: “Direct Liquid Chromatography/Mass Spectrometry Analysis for Complete Characterization of Recombinant Adeno-Associated Virus Capsid Proteins,” Human Gene Therapy Methods, 2017
[11] Wohlrab et al.: “Tracking Host Cell Proteins During Biopharmaceutical Manufacturing: Advanced Methodologies to Ensure High Product Quality,” American Pharmaceutical Review, 2018
[12] Goey et al.: “Host cell protein removal from biopharmaceutical preparations: Towards the implementation of quality by design,” Biotechnology Advances, 2018
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.